Epifluorescence Microscopy
The platform has two widefield microscopes for the acquisition of material fixed in fluorescence, a widefield microscope equipped with a colour camera, a routine fluorescence microscope for the observation of living cells and an analysis computer equipped with Fiji and Icy software. This equipment is reserved for the internal use of UMR7216 members.

Artistic image of jellyfish, referring to the bioluminescent jellyfish from which GFP (Green Fluorescent Protein) is extracted that has revolutionised the world of imaging.
© Image par Carol-Ann Bussières de Pixabay
Leica DMI-6000B "Oldelaf"
Inverted epifluorescence microscope
X, Y and Z piloted
Filters and objectives piloted
Camera:
Camera CCD -30ºC regulated
CoolSNAP HQ2 14bits
Image size: 1392×1040 pixels
Pixel size: 6.45 x 6.45μm
Illumination:
Visible light (halogen)
CoolLED pE-300 White Series Fluorescent Lamp (LED, >25000h)
Acquisition software:
Metamorph with Multi-Dimensional Acquisition module
Objectives:
| Obj Mag. | N.A | Quality | Transmission Contrast | Imm. | Pixel size (binning 1) | Max resolution at 488nm |
| 10X | 0,25 | N PLAN | PH1 | Air | 0,645 μm | 1191 nm |
| 20X | 0,35 | N PLAN | PH1 | Air | 0,3225 μm | 850 nm |
| 40X | 0,6 | HCX PL Fluotar | PH2 | Air | 0,161 μm | 496 nm |
| 40X | 1,25-0,75 | HC PL APO CS | – | Oil | 0,161 μm | 229 nm |
| 63X | 1,40 | HCX PL APO CS | PH3 | Oil | 0,102 μm | 213 nm |
| 100X | 1,40 | HCL PL APO CS | PH3 | Oil | 0,0645 μm | 213 nm |
Filters :
| Filter name | Colour | Excitation Filter BP | Dichroic Mirror | Suppression Filter BP |
| A4 | Blue | 360/40 | 400 | 470/40 |
| GFP | Green | 470/40 | 500 | 525/50 |
| Y3 | Cy3 (orange) | 545/40 | 565 | 610/75 |
| TX2 | Red | 560/40 | 595 | 645/75 |
| Y5 | Cy5 (Far red) | 620/60 | 660 | 700/75 |
| Analyzer cube | – | – | – | Analyzer |
Leica DMI-6000B "Lenny"
Inverted epifluorescence microscope
Controlled in X, Y and Z
Controlled filters and lenses
Camera:
Camera CCD -12ºC regulated
Teledyne Photometrics RETIGA R6
Image size: 2688×2200 pixels
Pixel size: 4.54 x 4.54μm
Illumination:
Visible light (halogen)
CoolLED pE-300 White Series Fluorescent Lamp (LED, >25000h)
Acquisition software:
Metamorph with Multi-Dimensional Acquisition module
Objectives:
| Mag. | N.A | Quality | Trans Contrast | Imm. | Pixel size (binning 1) | Max resolution at 488 nm |
| 2,5X | 0,07 | HC FL PLAN | – | Air | 1.816 μm | 4,2 µm |
| 20X | 0,7 | HCL PL APO CORR | – | Oil/Gly | 0,227 μm | 425 nm |
| 40X | 1,25-0,75 | HC PL APO | – | Oil | 0,1135 μm | 238 nm |
| 63X | 1,40-0,6 | HCX PL APO | – | Oil | 0,072 μm | 212 nm |
| 100X | 1,40-0,7 | HCX PL APO | – | Oil | 0,0454 μm | 212 nm |
Filters :
| Name | Colour | Excitation Filter BP | Dichroic Mirror | Suppression Filter BP |
| XF131 | Blue | 387/28 | 410 | 450/65 |
| QMAX green | Green | 450-490 | 500 | 510-560 |
| N3 | Cy3 (orange) | 546/12 | 565 | 600/40 |
| TX2 | Red | 560/40 | 595 | 645/75 |
| Far Red | Cy5 | 620-60 | 660 | 700-75 |
| Analyzer cube | – | – | – | Analyzer |
Leica DM IL LED (Live)
Inverted epifluorescence microscope
Camera:
Camera CCD -60ºC regulated
Hamamatsu digital camera C4742-98-24ERG 12 or 14 bits
Image size: 1344×1024
Pixel size: 6.45 x 6.45μm
Illumination:
Visible light (halogen)
Leica fluorescent lamp (Mercury Halides – 3000h max)
Objectives:
| Mag. | N.A | Quality | Transmission Contrast |
Imm. | Pixel size (binning 1) |
Max resolution at 488 nm |
| 10X | 0,25 | N PLAN | PH1 | Air | 0,645 μm | 1,2 µm |
| 20X | 0,35 | N PLAN | PH1 | Air | 0,322 μm | 850 nm |
| 40X | 0,55 | CORR | PH2 | Air | 0,161 μm | 541 nm |
Filtres :
| Name | Colour | Excitation Filter BP | Dichroic Mirror | Suppression Filter BP |
| B/G/R | Blue/Green/Red | 420/30 ; 495 /15 ; 570/20 | 415 ; 510 ; 590 | 465/20 ; 530 /30 ; 640/40 |
| GFP ET | Green | |||
| TX ET | Red |
Leica DMRA2 (Color)
Upright epifluorescence microscope
Controlled in X, Y and Z
Camera:
CCD digital color camera 5Mpixels Leica DFC 450C cooled (Δ -20ºC compared to ambient)
Image size: 2560×1920
Pixel size: 3.4 x 3.4μm
Illumination:
Visible light (halogen)
Fluorescent lamp not installed
Objectives:
| Mag. | N.A | Quality | Transmission Contrast |
Imm. | Pixel size (binning 1) |
Max resolution at 488 nm |
| 10X | 0,30 | HC PL Fluotar | – | Air | 0,34 μm | 992 nm |
| 20X | 0,50 | HCX PL Fluotar | – | Air | 0,17 μm | 595 nm |
| 40X | 0,75 | HCX PL Fluotar | – | Air | 0,085 μm | 397 nm |
| 100X | 1,40-0,7 | HCX PL APO CS | – | Oil | 0,034 μm | 212 nm |
Workstation "Angus"
A workstation for image analysis is available. This workstation is equipped with the free software FIJI, IMAGEJ, ICY.
Read more
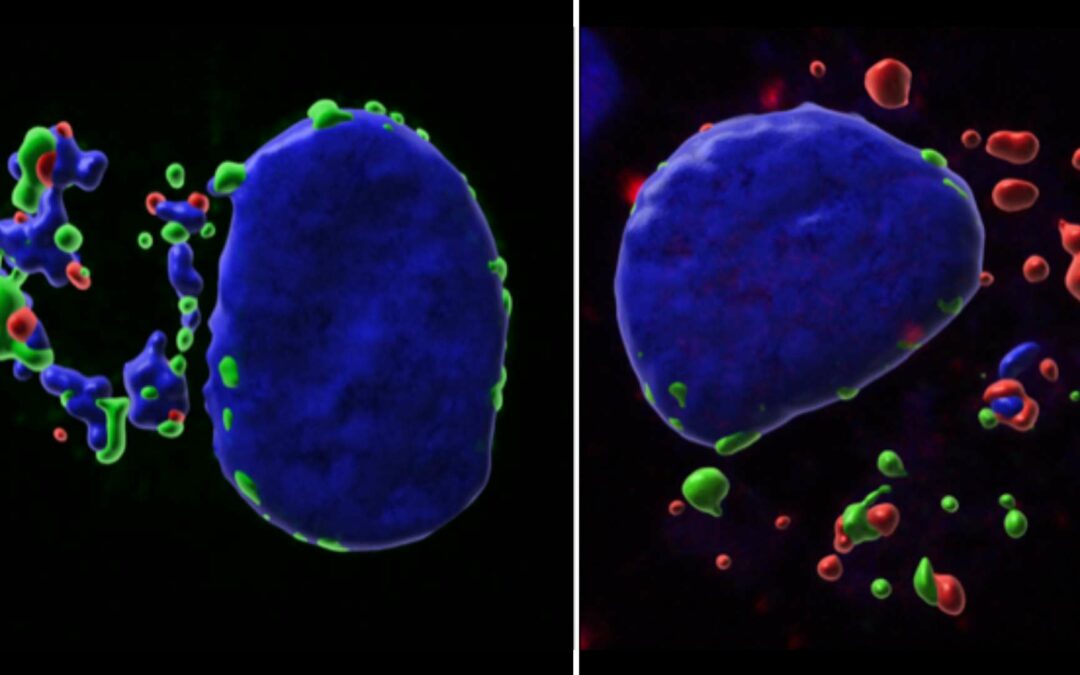
Theileria annulata and Cancer: a parasite strategy revealed!

Laetitia Aubry seminar – April 24, 2024
Wednesday, April 24, 2024, at 11:00 am. Laetitia Aubry Université d’Evry / I-StemInvited by Valérie Drouet “Harnessing pluripotent stem cells derivatives from patients with Wolfram syndrome to reveal pathological mechanisms and identify potential therapeutic chemical...
Moussa Benhamed seminar – April 17, 2024
Wednesday, April 17, 2024, at 11:00 am. Moussa Benhamed Institute of Plant Sciences, Paris SaclayInvited by Valérie Mezger “Exploring the chromatin-based regulation of enhancer-promoter contact and its impact on gene expression” The seminar will take place in the...
Professional afterwork – April 22, 2024
Do you know what else exists outside academia?Come to talk about it with our two guests on Monday, April 22, 2024, at 6.15 pm. Where? Epigenetics and Cell Fate Center (EDC)Université Paris Cité - 35 Rue Helene Brion 75013 Paris – Lamarck B building - Hall (Upper...
