DNA methylation and genome maintenance
When studying a rare disease sheds new light on the field of DNA methylation
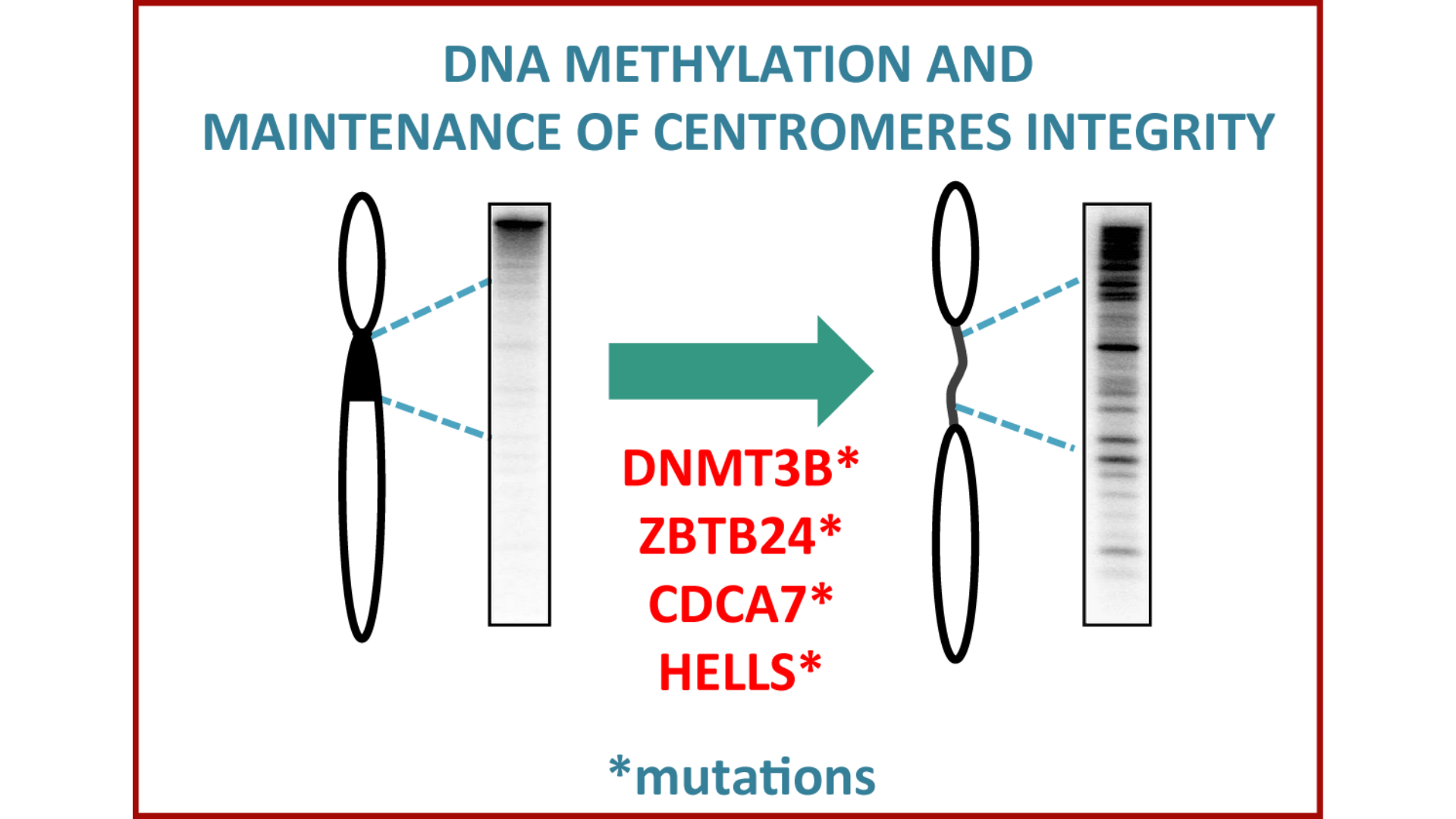
Mutation in the 4 four genes induces centromeres hypomethylation
© EDC
This raised important questions regarding their function, their direct or indirect role in pathways to DNA methylation, their genomic targets and the impact of their mutations on transcriptome, epigenome and cell fate. We aim at providing a comprehensive and integrated view of the consequences of perturbed DNA methylation in cells from patients and mouse models with important consequences to (i) understand the genotype/phenotype relationship in such a complex monogenic and epigenetic disease, (ii) establish biomarkers to aid diagnosis and prioritize patients for mutation screening and appropriate management, and (iii) in a more basic research orientation, to shed new light on the molecular mechanisms involved in establishment and maintenance of DNA methylation patterns at DNA repeats and unique genes, and on the consequences for long and short, coding and non-coding transcriptional output, with added relevance to other physiopathological contexts.
Related publications:
- Gatto S, Gagliardi M, Franzese M, Leppert S, Papa M, Cammisa M, Grillo G, Velasco G, Francastel C, Toubiana S, D’Esposito M, Angelini C, Matarazzo MR. ICF-specific DNMT3B dysfunction interferes with intragenic regulation of mRNA transcription and alternative splicing . Nucleic Acids Res. 2017 Mar 9; 45(10):5739-5756. PMID: 28334849
- Sagie S, Toubiana S, Hartono S, Katzir H, Tzur-Gilat A, Havazelet S, Francastel C, Velasco G, Chedin F, Selig S. Telomeres in ICF syndrome cells are vulnerable to DNA damage due to elevated DNA:RNA hybrids. Nat Commun. 2017 Jan 24;8:14015. PMID: 28117327
- Sterlin D, Velasco G, Moshous D, Touzot F, Mahlaoui N, Fischer A, Suarez F, Francastel C, Picard C. Genetic, Cellular and Clinical Features of ICF Syndrome: a French National Survey . J Clin Immunol. 2016 Feb;36(2):149-59. PMID: 26851945
- Thijssen PE, Ito Y, Grillo G, Wang J, Velasco G, Nitta H, Unoki M, Yoshihara M, Suyama M, Sun Y, Lemmers RJ, de Greef JC, Gennery A, Picco P, Kloeckener-Gruissem B, Güngör T, Reisli I, Picard C, Kebaili K, Roquelaure B, Iwai T, Kondo I, Kubota T, van Ostaijen-Ten Dam MM, van Tol MJ, Weemaes C, Francastel C, van der Maarel SM, Sasaki H. Mutations in CDCA7 and HELLS cause immunodeficiency-centromeric instability-facial anomalies syndrome. Nat Commun. 2015 Jul 28;6:7870. PMID: 26216346
- Walton E, Francastel C, Velasco G. Dnmt3b prefers germ line genes and centromeric regions: lessons from ICF and cancer and implications for diseases. Biology (Basel). 2014 Sep 5;3(3):578-605. PMID: 25198254
- Velasco G, Walton EL, Sterlin D, Hédouin S, Nitta H, Yuya I, Fouyssac F, Mégarbané A, Sasaki H, Picard C, Francastel C. Germline genes hypomethylation and expression define a molecular signature in peripheral blood of ICF patients: implications for diagnosis and etiology. Orphanet J Rare Dis. 2014 Apr 17;9(1):56. PMID: 24742017
- Nitta H, Unoki M, Ichiyanagi K, Kosho T, Shigemura T, Takahashi H, Velasco G, Francastel C, Picard C, Kubota T, Sasaki H. Three novel ZBTB24 mutations identified in Japanese and Cape Verdean type 2 ICF syndrome patients. J Hum Genet. 2013 Jul;58(7):455-60. PMID: 23739126
- Walton EL, Francastel C, Velasco G. Maintenance of DNA methylation: Dnmt3b joins the dance. Epigenetics. 2011 Nov;6(11):1373-7. PMID: 22048250
- Velasco G, Hubé F, Rollin J, Neuillet D, Philippe C, Bouzinba-Segard H, Galvani A, Viegas-Péquignot E, Francastel C. Dnmt3b recruitment through E2F6 transcriptional repressor mediates germ-line gene silencing in murine somatic tissues. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9281-6. PMID: 20439742
Read more

Welcome to Léa
Léa joins the team as a research assistant. After completing a master's degree in virology, she worked in Strasbourg on grapevine viruses, then on characterizing mRNA degradation in plants at the Institute of Plant Molecular Biology (IBMP). In the Polo team, Léa will...

Sophie Polo receives an Impulscience® grant from the Fondation Bettencourt Schueller
Sophie Polo has been awarded an Impulscience® grant to fund a research project on the establishment and maintenance of the inactive X chromosome in response to DNA breaks. This is wonderful news for the lab ! We thank the Fondation Bettencourt Schueller for their...

Welcome to Léa, new engineer in the team!
Léa joins the lab as a research assistant. She holds a Master's degree in Molecular and Cellular Biology from Sorbonne University. She will contribute to investigate DNA methylation maintenance mechanisms in response to UV damage in mammalian cells. Léa Girard À lire...

Well done, Dr Mori!
Margherita successfully defended her PhD on DNA methylation maintenance in response to UV damage. Brava! Margherita and her thesis jury. From left to right: Sophie Polo, Sandra Duharcourt (on screen), Déborah Bourc'his, Margherita Mori, Nataliya Petryk, Jean Molinier,...
