Djeghloul group
Mitotic Inheritance of Cell identity
Our team investigates how cell identity and epigenetic memory are sustained or altered during cell division, particularly at mitosis, in the context of stemness and human B lymphoid lineage. This fundamental question is a central challenge in cell biology with key implications for human health and diseases.
B lymphocyte cells arise from multipotent hematopoietic stem cells (HSCs) through a succession of lineage restriction, cell determination and specialisation programs, that take place over multiple rounds of cell cycle divisions as they mature. Stable maintenance of B cell lymphoid identity, as HSCs differentiate, is essential for continuous B cell production and function ensuring immune system integrity throughout life. Disruptions in this process may lead to immunological disorders and hematopoietic malignancies.
During cell division both genetic and epigenetic materials need to be copied and accurately conveyed to newly divided daughter cells. During mitosis, chromatin undergoes extensive changes: chromosomes highly condense, transcription is largely reduced, and many DNA-binding factors are evicted from mitotic condensed chromosomes. Consequently, mitosis furnishes a very challenging chromatin environment to sustain cell identity. Although many DNA-associated factors are displaced from mitotic chromosomes, others remain chromosome-bound, and are able to occupy at least a subset of the genomic sites bound during interphase, so-called “mitotic bookmarking” (Figure 1). These factors help to reinstate the gene transcription programs in the daughter cells, thereby ensuring that cell identity is accurately conveyed. This concept has been mainly explored in embryonic stem cells that need a constant balance between cell identity maintenance and plasticity. Cellular memory requirements may therefore differ in more committed specialised cells that require stable maintenance of lineage identity throughout life in order to ensure lineage stability and specificity of function. Beside DNA-bound factors that can reactivate gene expression post-mitosis, it is also becoming evident that the underlying chromatin state, including repressive heterochromatin components that are stably maintained throughout mitosis, is also a key parameter in sustaining cellular memory.
To uncover the molecular basis of cellular memory in a systematic and unbiased manner, the lab employs innovative approaches combining mitotic chromosome flow-sorting with high-throughput proteomics and multi-omics analyses. This enables quantitative assessment of DNA-bound proteins, chromatin features, and RNAs on native mitotic chromatin, as well as functional interrogation of their role in transcriptional and epigenetic memory. Our goal is to decipher the molecular mechanisms that ensure the stable maintenance of B lineage identity through cell division and to understand their impact on immune gene regulation and B cell function.
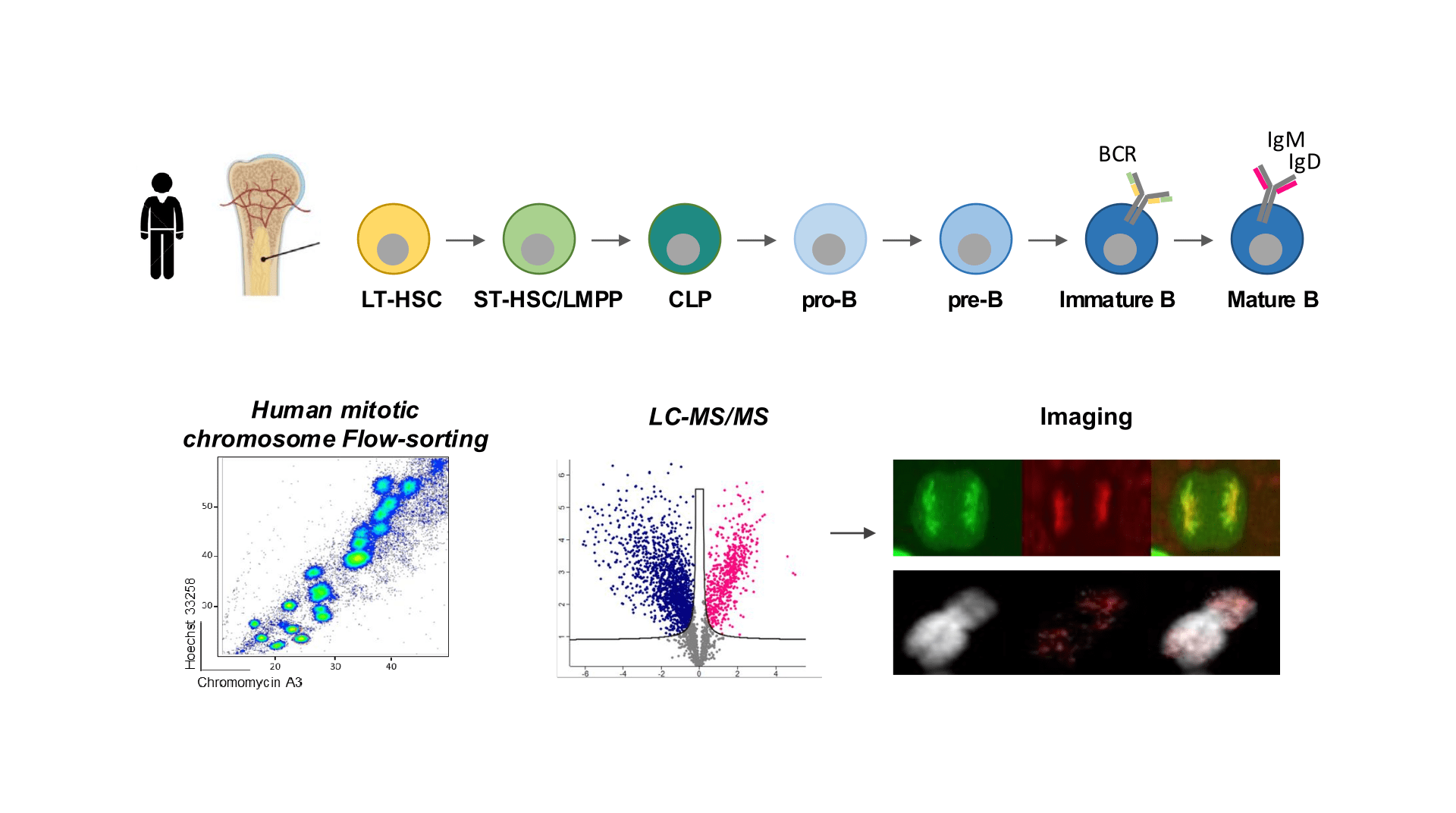
Identifying factors conveying B lymphoid cell identity through cell division
Description
B lymphoid differentiation as a model to study mitotic memory in the context of cell fate commitment and differentiation.
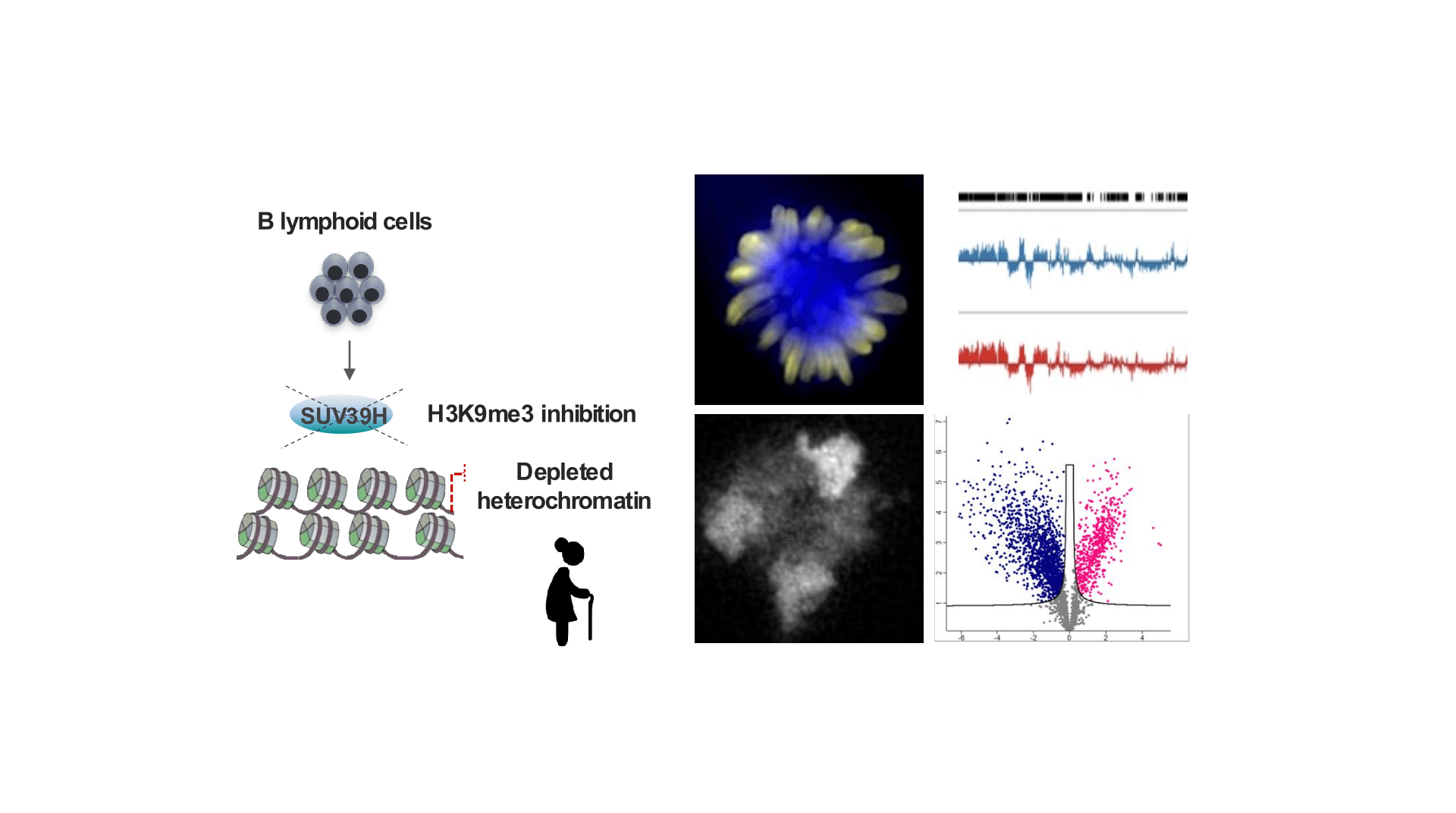
Interdependence between chromatin state and cellular memory
Description
- Role of H3K9me3 in conveying identity of B lymphoid lineage
- Impact of age-related changes in heterochromatin on cell identity and genomic fidelity inheritance in normal and pathological B lymphopoiesis
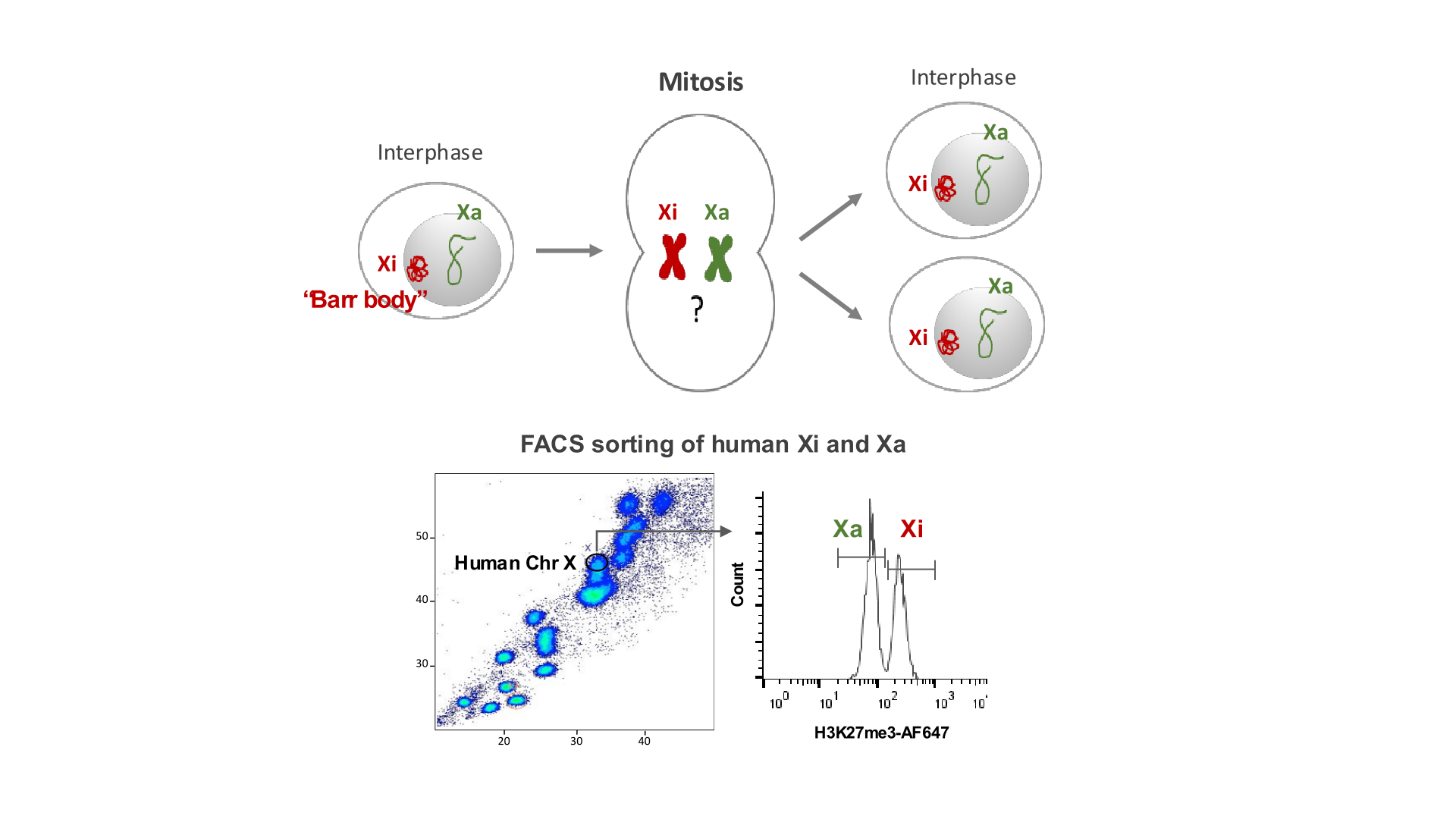
Studying factors that convey the epigenetic memory of XCI in human B lymphocytes
Description
The X chromosome hosts a large number of inflammatory and immunity-related genes, and increasing evidence indicates that X chromosome inactivation (XCI) maintenance is essential for normal immune pathways and B lymphocyte development. XCI is a paradigm of epigenetic inheritance and offers a unique opportunity to study how epigenetic signatures are clonally propagated through cell division and reinstated in daughter cells.
In the lab, we use chromosome flow sorting to isolate the human active and inactive X chromosomes from B lymphocytes, identify their chromatin-bound factors, and assess their role in X-linked immune gene dosage and sex-biased immune response.
Publications
PUBLICATIONS:
DJEGHLOUL D*, Cheriyamkunnel S, Patel B, Kramer H, Montoya A, Brown KE, Whilding C, Nesterova T, Wei G, Brockdorff N, Grządzielewska I, Karayol R, Akhtar A, Merkenschlager M and Fisher AG*. Hbo1 and Msl complexes preserve differential compaction and H3K27me3 marking of active and inactive X chromosomes during mitosis. Nat Cell Biol. 2025. Sep;27(9):1482-1495. doi: 10.1038/s41556-025-01748-0. PMID: 40921734
Dimond A, Gim DH, Ing-Simmons E, Kramer H, DJEGHLOUL D, Montoya A, Patel B, Cheriyamkunnel S, Brown KE, Shliaha P, Vaquerizas JM, Merkenschlager M and Fisher AG*. PBK/TOPK mediates Ikaros, Aiolos and CTCF displacement from mitotic chromosomes and alters chromatin accessibility at selected C2H2-zinc finger protein binding sites. Nat Commun. 2025. Sep 23;16(1):8348. doi: 10.1038/s41467-025-63740-4. PMID: 40987773
DJEGHLOUL D*, Dimond A, Cheriyamkunnel S, Kramer H, Patel B, Brown K, Montoya A, Whilding C, Wang YF, Futschik ME, Veland N, Montavon T, Jenuwein T, Merkenschlager M, Fisher AG*. Loss of H3K9 tri-methylation impacts mitotic chromosome compaction and transcription factor retention. Nat Struct Mol. 2023. Apr;30(4):489-501. doi: 10.1038/s41594-023-00943-7. PMID: 36941433.
DJEGHLOUL D, Patel B, Kramer H, Dimond A, Whilding C, Brown K, Kohler AC, Feytout A, Veland N, Elliott J, Bharat TAM, Tarafder AK, Löwe J, Ng BL, Guo Y, Guy J, Huseyin MK, Klose RJ, Merkenschlager M, Fisher AG*. Identifying proteins bound to native mitotic ESC chromosomes reveals chromatin repressors are important for compaction. Nat Commun. 2020. Aug 17;11(1):4118. doi: 10.1038/s41467-020-17823-z. PMID: 32807789.
Cuartero S, Weiss FD, Dharmalingam G, Guo Y, Ing-Simmons E, Masella S, Robles-Rebollo I, Xiao X, Wang YF, Barozzi I, DJEGHLOUL D, Amano MT, Niskanen H, Petretto E, Dowell RD, Tachibana K, Kaikkonen MU, Nasmyth KA, Lenhard B, Natoli G, Fisher AG, Merkenschlager M*. Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation. Nat Immunol. 2018. Sep;19(9):932-941. doi: 10.1038/s41590-018-0184-1. PMID: 30127433.
DJEGHLOUL D, Kuranda K, Kuzniak I, Barbieri D, Naguibneva I, Choisy C, Bories JC, Dosquet C, Pla M, Vanneaux V, Socié G, Porteu F, Garrick D, Goodhardt M. Age-Associated Decrease of the Histone Methyltransferase SUV39H1 in HSC perturbs heterochromatin and B lymphoid differentiation. Stem Cell Reports. 2016. Jun 14;6(6):970-984. doi: 10.1016/j.stemcr.2016.05.007. PMID: 27304919.
Lescale C#, Schenten V#, DJEGHLOUL D, Bennabi M, Gaignier F, Vandamme K, Strazielle C, Kuzniak I, Petite H, Dosquet C, Frippiat JP, Goodhardt M. Hind limb unloading, a model of spaceflight conditions, leads to decreased B lymphopoiesis similar to aging. FASEB J. 2015. Feb;29(2):455-63. doi: 10.1096/fj.14-259770. PMID: 25376832.
Armand AS, Laziz I, DJEGHLOUL D, Lécolle S, Bertrand AT, Biondi O, De Windt LJ, Chanoine C. Apoptosis-inducing factor regulates skeletal muscle progenitor cell number and muscle phenotype. PLoS press. 2011. 6(11):e27283. doi: 10.1371/journal.pone.0027283. PMID: 22076146.
BOOK CHAPTERS:
Garrick D, David A, Freitas C, DJEGHLOUL D, Goodhardt M. Noncoding RNA and Epigenetic Change in Hematopoietic Stem Cell Aging. Springer International Publishing, Handbook of Immunosenescence, 2019, p. 1011-1038, doi: 10.1007/978-3-319-99375-1_99.
Garrick D, DJEGHLOUL D, Kuranda K, Goodhardt M. Aging of Human Haematopoietic Stem Cells. Springer, Stem Cell Aging: Mechanisms, Consequences, Rejuvenation, 2015, p. 127-147, doi : 10.1007/978-3-7091-1232-8_7.
Contact
dounia-zede.djeghloul@cnrs.fr
Members
Funding
À lire aussi

Welcome to Léa
Léa joins the team as a research assistant. After completing a master's degree in virology, she worked in Strasbourg on grapevine viruses, then on characterizing mRNA degradation in plants at the Institute of Plant Molecular Biology (IBMP). In the Polo team, Léa will...

Sophie Polo receives an Impulscience® grant from the Fondation Bettencourt Schueller
Sophie Polo has been awarded an Impulscience® grant to fund a research project on the establishment and maintenance of the inactive X chromosome in response to DNA breaks. This is wonderful news for the lab ! We thank the Fondation Bettencourt Schueller for their...

Welcome to Léa, new engineer in the team!
Léa joins the lab as a research assistant. She holds a Master's degree in Molecular and Cellular Biology from Sorbonne University. She will contribute to investigate DNA methylation maintenance mechanisms in response to UV damage in mammalian cells. Léa Girard À lire...

Well done, Dr Mori!
Margherita successfully defended her PhD on DNA methylation maintenance in response to UV damage. Brava! Margherita and her thesis jury. From left to right: Sophie Polo, Sandra Duharcourt (on screen), Déborah Bourc'his, Margherita Mori, Nataliya Petryk, Jean Molinier,...



